How To Draw A Chair Conformation For A Compound With 2 O's
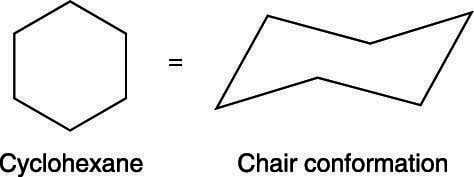
The chair conformation of cyclohexane
The importance of drawing chairs
Many students detect that cartoon chairs can exist somewhat difficult at outset, simply mastering the right drawing of this conformation is essential for your success in organic chemistry. To begin, start past drawing two lines that are parallel to each other but non perfectly horizontal, as shown here. Next, add a downwardly-pointing V tip to 1 end (this is the tail of the chair). Finally, add together an upwards-pointing V tip to the other end (this is the nose of the chair). 
The steps involved in cartoon the chair conformation of cyclohexane
Axial hydrogens, equatorial hydrogens
A cyclohexane chair contains two kinds of hydrogens — axial hydrogens and equatorial hydrogens. Axial hydrogens are those hydrogens that stick direct upwardly or straight down parallel to an imaginary axis through the chair; equatorial hydrogens are hydrogens that stick out along the equator of the chair. Both types are shown here. 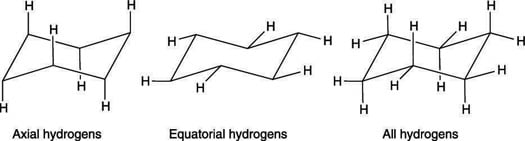
The axial and equatorial hydrogens on cyclohexane
In drawing the hydrogens on a chair cyclohexane, it's often easiest to draw the axial hydrogens outset. At any bespeak on the chair that sticks up, put the centric hydrogen sticking straight upwardly; at any signal on the chair that sticks downwards, draw the axial hydrogen direct down. After the axial hydrogens are drawn, adding in the equatorial hydrogens around the equator of the chair is a adequately straightforward job.
The ring flip
At room temperature, cyclohexane doesn't stay in one-chair conformation, simply apace interconverts into an alternative chair conformation with a band flip, shown hither. 
The ring flip of cyclohexane
When a ring flip doesn't alter the molecule
With unsubstituted cyclohexane (a cyclohexane that has just hydrogens attached to it), undergoing a ring flip doesn't modify the molecule. With substituted cyclohexanes, notwithstanding, the ii chair conformers may non exist identical. For case, isopropylcyclohexane is shown here. One chair conformer puts the isopropyl grouping in the axial position. Later undergoing a ring flip, the isopropyl becomes equatorial. 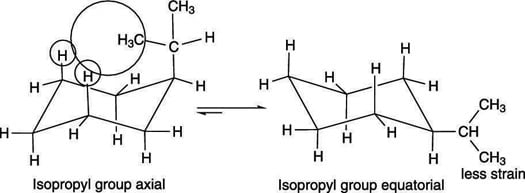
The chair conformers of isopropylcyclohexane
Band flips change all axial positions to equatorial and all equatorial positions to centric.
These two conformers are not identical, and they don't have the same energy. When a big group is axial, the large grouping invades the space of the hydrogens on carbons two positions away, introducing 1,3-diaxial strain. This interaction increases the free energy of the centric conformer. Therefore, every bit a dominion, large groups prefer to be equatorial because this conformation has no i,three-diaxial strain — equally shown in the figure, the right-pointing arrow is longer, indicating the direction of the equilibrium favors the more stable chair conformation.About This Article
This article can exist found in the category:
- Chemical science ,
How To Draw A Chair Conformation For A Compound With 2 O's,
Source: https://www.dummies.com/article/academics-the-arts/science/chemistry/how-to-draw-the-chair-conformation-of-cyclohexane-146367
Posted by: deloachcrehose.blogspot.com


0 Response to "How To Draw A Chair Conformation For A Compound With 2 O's"
Post a Comment